Table of Contents
ToggleEnsuring Quality: Strategies of Derma Roller Wholesalers
This article delves into how derma roller wholesalers ensure the quality of their products, covering key aspects like high-quality raw materials, rigorous manufacturing standards, and comprehensive quality control inspections. By following these guidelines, wholesalers can guarantee the safety, effectiveness, and reliability of their derma rollers, benefiting both themselves and their customers.
Furthermore, the article highlights the importance of ongoing training and education for production staff, as well as the significance of adhering to international safety and compliance certifications. By implementing advanced packaging methods, proper sterilization processes, and transparent vendor audits, wholesalers of micro derma roller can maintain high product standards and build trust with consumers, ultimately establishing themselves as reputable and reliable brands in the market.
Key Points
- High-quality raw materials are crucial
- Rigorous manufacturing standards are essential
- Comprehensive quality control inspections are key
- Proper sterilization processes must be followed
- Adherence to international safety and compliance certifications
- Utilization of advanced packaging methods is important
- Transparent vendor audits and documentation are necessary
- Ongoing training and education for production staff

Picking High-Quality Raw Products for Derma Rollers
In my extensive experience as a derma roller wholesaler, I have actually found that choosing high-grade basic materials is crucial to ensuring the total quality of our items. This process includes meticulous sourcing and comprehensive vetting of distributors to assure that the materials used meet our rigid criteria.
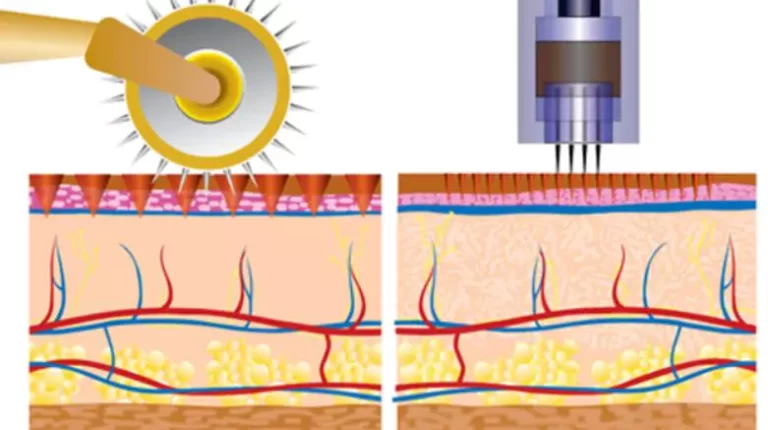
The primary elements of a derma roller consist of the needles, the roller, and the care. Each of these parts needs mindful factor to consider:
| Part | Product | Key high-quality indicators |
|---|---|---|
| Needles | Medical-grade stainless-steel or titanium | Resilience, rust resistance, and biocompatibility |
| Roller | Top-quality plastic or material | Toughness, smooth turning, and chemical resistance |
| Deal with | Ergonomic, non-slip product | Comfort, grip, and balance |
For the needles, we exclusively use medical-grade stainless steel or titanium, renowned for their longevity, deterioration resistance, and biocompatibility. These products are necessary to protect against negative skin reactions and ensure the long-term effectiveness of the derma rollers.
The roller itself is normally made from top-quality plastic or resin. We prioritize products that use superior stamina, smooth turning, and chemical resistance to preserve the honesty of the device throughout use and cleaning.
The handle has to be made from an ergonomic, non-slip material to give individuals a comfortable and secure grasp. This is critical for the precision and safety of the derma rolling procedure.
By adhering to these requirements and regularly evaluating product performance with responses and screening, we make sure that our derma rollers not only fulfill but exceed market expectations.
Applying Strenuous Production Specifications
To ensure the best quality of derma rollers, it is essential to apply extensive manufacturing standards throughout the production process. These criteria begin with the choice of products and reach every phase of production.
First, it is vital to keep a controlled production environment. This includes using tidy areas that satisfy rigorous tidiness and contamination control protocols. By controlling the environment, we can reduce the risk of presenting impurities or pollutants throughout production.
Next, accuracy in manufacturing is critical. Utilizing advanced equipment and modern technology makes certain that each derma roller is generated to exact specifications. This includes the exact placement and development of needles, which are vital for the device’s efficiency and safety. Normal calibration of equipment is needed to maintain these high standards.
An additional key element is the application of standard operating procedures (SOPs). These procedures offer comprehensive instructions for every step of the production procedure, ensuring consistency and lowering the possibility of mistakes. SOPs should be consistently examined and updated to include the newest industry methods and regulatory demands.
In addition, labor force training plays a considerable role in maintaining production standards. Manufacturing personnel should be thoroughly educated in SOPs and the particular demands of derma roller manufacturing. Continuous education, learning, and training programs help keep personnel informed about new innovations, methods, and regulatory adjustments.
Finally, documentation and traceability are vital elements of strenuous production standards. Every batch of derma rollers should be recorded carefully, consisting of information about the raw products utilized, the production problems, and any assessments or tests performed. This document ensures traceability and responsibility, enabling quick identification and resolution of any concerns that may arise.
Performing Comprehensive Quality Assurance Inspections
Making certain the top quality of derma rollers is paramount for dealers, and this demands performing thorough top quality control inspections at different stages of the manufacturing procedure. These examinations are created to detect and remedy any defects or disparities that may develop, therefore ensuring the effectiveness and security of the final item.
Quality control starts with the assessment of incoming raw products. Each batch of products is extensively examined for pureness, strength, and conformity with predefined requirements. This initial step is crucial to making certain that only the most effective products are used in the manufacturing procedure.
During manufacturing, several checkpoints are established to keep track of the production procedure. These checkpoints generally include the following:
| Checkpoint | Assessment Standard | Regularity |
|---|---|---|
| Needle Intensity | Ensuring needles are consistently sharp and without problems | Every 100 units |
| Roller Positioning | Confirming that needles are correctly lined up and spaced | Every 200 units |
| Handle Stability | Examining the stamina and durability | Every 300 units |
Post-production, a final inspection is executed to make certain that the derma rollers fulfill all high quality and security criteria. This last inspection consists of a thorough assessment of the completed product for any type of prospective issues, such as needle misalignment, contamination, or architectural weak points. Products that do not satisfy the well-established requirements are promptly rejected and returned for re-evaluation or disposal.
Additionally, advanced technologies such as digital imaging and automatic inspection systems are progressively being used to improve the precision and effectiveness of quality assurance examinations. These modern technologies make it possible for the detection of even the tiniest problems that might not show up to the naked eye, thereby further guaranteeing the reliability of the derma rollers.
By carrying out a durable, high-quality control system, derma roller dealers can maintain high criteria and ensure that their items are secure, efficient, and of the highest possible quality. This not only shields the end-users but also aids in constructing a reliable brand on the market.
Making Certain Appropriate Sterilization Processes
Making certain appropriate sanitation procedures is an essential action in preserving the quality, safety, and security of derma rollers. The main goal is to remove any potential pollutants that could pose a danger to individuals. This process starts with the option of ideal sterilization methods, which commonly consist of gamma radiation, ethylene oxide (ETO) gas, or autoclaving. Each method has its very own advantages and is picked based upon the details products and layout of the derma rollers.
Prior to the sterilization process starting, all derma rollers have to be thoroughly cleansed to remove any kind of recurring manufacturing debris. This is generally accomplished with a combination of ultrasonic cleaning and rinsing with medical-grade services. The relevance of this pre-cleaning step can not be overemphasized, as any type of deposit left on the devices can reduce the effectiveness of the sterilization procedure.
As soon as they are cleansed, the derma rollers are meticulously packaged in sterilizable materials. The product packaging must be made to maintain sterility up until the item is opened by the end user. This usually involves the use of medical-grade bags or sore packs that are secured to avoid contamination.
The selected sterilization approach is then used. As an example, when using gamma radiation, the packaged derma rollers are exposed to a detailed dose of gamma rays, which pass through the product packaging and get rid of bacteria, infections, and various other pathogens. This technique is very efficient and does not leave any type of chemical deposit, making it a recommended selection for numerous manufacturers.
Alternatively, ETO gas sanitation involves subjecting the derma rollers to ethylene oxide gas in a regulated setting. This approach is especially valuable for materials that can not endure the high temperature levels of autoclaving. The ETO gas penetrates the product packaging and effectively sanitizes the components. However, it is important to make certain that any kind of recurring gas is properly removed prior to the items being shipped, as ETO is toxic in high focus.
Autoclaving, which utilizes high-pressure saturated steam, is one more usual technique, specifically for elements that can endure high temperature levels. This procedure is extremely reliable for disinfecting steel parts, which are often found in derma rollers. The trick to effective autoclaving is accurate control of temperature level, pressure, and time to ensure complete sterilization.
Post-sterilization, it is vital to verify the performance of the procedure. This is done via various testing methods, including organic signs and chemical integrators, which verify that the sterilization criteria have actually been satisfied. Additionally, regular audits and validation research studies are performed to ensure ongoing compliance with established sterilization standards.
By adhering to stringent sanitation methods, derma roller dealers can make sure that their items are secure and all set for consumer usage. These steps are basic in developing trust with consumers and maintaining the stability of the brand name.
Sticking To International Safety, Security and Compliance Certifications
Making sure the top quality of derma rollers entails strict adherence to various international safety and compliance accreditations. These accreditations are vital as they provide a standardized structure that ensures item security, effectiveness, and reliability. By sticking to these criteria, dealers can assure their customers and end-users of the premium quality, safety and security of their derma rollers.
One of the most recognized accreditations is ISO 13485, which specifies requirements for a detailed, high-quality monitoring system for the style and manufacture of clinical tools. This typically ensures that derma rollers are consistently produced and regulated to fulfill the appropriate safety and performance standards. Executing ISO 13485 needs regular audits and constant enhancement procedures, thus promoting an atmosphere of ongoing high quality assurance.
An additional critical accreditation is CE Marking, which shows that a product complies with the essential health, safety, security, and environmental management demands of the European Economic Area (EEA). For derma rollers, acquiring CE marking includes strenuous testing and documents to confirm that the item satisfies all appropriate EU regulations. This certification not only assists in access to the European market but also works as a mark of top quality, safety, and security globally.
Along with ISO 13485 and CE marking, derma roller dealers also need to consider FDA clearance if they prepare to disperse their items in the USA. The FDA’s 510(k) procedure requires manufacturers to demonstrate that their derma rollers are considerably comparable to a legally marketed device. This entails in-depth documentation and typically medical data to sustain the safety, security, and efficiency of the item.
| Certification | Region | Secret Demands | Benefits |
|---|---|---|---|
| ISO 13485 | Global | Quality monitoring system for clinical devices | Makes sure consistent item quality, safety and security |
| CE Marking | European Economic Area | Conformity with EU health, safety, and ecological protection standards | Accessibility to the European market, international acknowledgment |
| FDA Clearance (510(k)) | United States | Considerable equivalence to a legitimately marketed device | Access to the US market, assurance of security and performance |
Furthermore, adherence to Great Production Practices (GMP) is necessary. GMP standards offer a framework for ensuring that items are consistently produced and regulated according to quality requirements. This entails in-depth paperwork for making procedures, regular examinations, and stringent control for manufacturing atmospheres to prevent contamination and ensure product uniformity.
In summary, by sticking to these international safety and conformity certifications, derma roller wholesalers can maintain high product standards, ensure client safety, and gain access to international markets. These accreditations not only reflect the dedication to high quality but also build trust with customers and regulatory bodies alike.
Utilizing Advanced Packaging Methods to Preserve Sterility
In the world of derma roller wholesaling, ensuring sterility is extremely important to preserving the quality and safety of the items. One of the important measures embarked on is the implementation of sophisticated packaging strategies. These techniques are developed to secure the derma rollers from contamination during storage and transit, thereby protecting their sterility until they reach the end consumer.
The packaging process starts with the selection of high-grade, medical-grade products that develop a barrier versus microbial seepage. These products usually consist of sterilizable plastics and aluminum foils that are resistant to leaks and splits. The stability of the packaging material is vital since any kind of breach can jeopardize the sterility of the derma rollers.
Moreover, the packaging setting is purely managed. Cleanrooms with HEPA filtration systems are used to reduce the existence of air-borne particles. In these settings, employees comply with stringent health methods, consisting of the use of gloves, masks, and protective garments, to avoid contaminating the items.
To enhance sterility, the packaging process often integrates sterilization approaches such as gamma irradiation or ethylene oxide gas. These techniques are effective in removing any type of recurring microorganisms that could have made it through the first manufacturing and product packaging phases. The option of a sterilization approach depends on the product compatibility and the particular requirements of the derma rollers.
Another element of innovative product packaging techniques is making use of hermetic securing. This procedure entails creating impermeable seals that prevent the ingress of contaminants. Hermetically secured plans are particularly reliable in maintaining sterility over extended periods, ensuring that the derma rollers continue to be secure and effective until they are opened up for use.
Furthermore, derma roller wholesalers often carry out tamper-evident features within the product packaging. These attributes give a visible sign if the plan has actually been opened up or tampered with, supplying an additional layer of safety, security, and assurance to both stores and consumers.
Advanced packaging strategies likewise consist of the use of desiccants and oxygen absorbers within the product packaging. These components help manage the interior atmosphere by reducing wetness and oxygen levels, which can contribute to microbial development. By preserving an optimal environment, the risk of contamination is substantially alleviated.
Ultimately, thorough labeling is an integral part of the packaging procedure. Labels include crucial information such as batch numbers, expiration dates, and sterilization information. This transparency enables traceability and makes certain that all events entailed in the supply chain are notified about the item’s problem and history.
Supplying Clear Derma Roller Vendor Audits and Documentation
Guaranteeing the high quality of derma rollers starts with a clear and detailed audit of vendors. Derma roller wholesalers should do thorough analyses of potential providers to confirm their capability to supply premium resources constantly. This consists of checking the vendor’s manufacturing processes, quality assurance systems, and compliance with international standards.
Secret components of a transparent vendor audit include:
| Audit Part | Summary | Frequency |
|---|---|---|
| Production Refine Review | Examine the production methods and modern technologies utilized by the provider to guarantee they fulfill certain top quality criteria. | Every year |
| Quality Assurance Solution | Assess the provider’s high-quality control procedures, consisting of testing and assessment protocols. | Biannually |
| Compliance Confirmation | Examine for adherence to global safety and regulatory criteria. | Quarterly |
| Supplier Certification | Guarantee vendors hold relevant certifications, such as ISO 13485, for clinical tools. | Upon first interaction and renewal |
Documents are just as essential to maintaining quality guarantees. Wholesalers should keep extensive records of all vendor audits, consisting of searches for, rehabilitative actions, and follow-up steps. This documentation needs to be conveniently accessible and regularly updated to reflect any kind of adjustments or renovations in the provider’s procedures.
Furthermore, it is very important to develop a clear and open line of interaction with suppliers. This involves sharing audit outcomes, offering feedback, and collaborating on required enhancements. By preserving a clear and participating relationship, derma roller wholesalers can guarantee that distributors are totally aligned with their quality assumptions and standards.
In summary, using transparent distributor audits and preserving comprehensive documents are vital actions in guaranteeing the constant quality of derma rollers. These techniques not only help in recognizing and reducing prospective risks, but likewise foster trust and reliability in between dealers and their providers.
Offering Ongoing Training, Education, and Learning for Production Personnel
Guaranteeing the high quality of derma rollers begins with the proficiency of the manufacturing team. To maintain high criteria, it is crucial to provide recurring training and education for all workers included in the manufacturing process. This commitment to continuous learning helps keep the group updated on the most recent market methods, technical improvements, and governing demands.
Normal training sessions must cover a selection of topics, consisting of:
| Training Topic | Regularity | Goal |
|---|---|---|
| Material Handling | Quarterly | Guarantee appropriate monitoring and use of raw materials to avoid contamination and defects. |
| Production Processes | Bi-Annually | Update the team on new methods and machinery to improve manufacturing efficiency and quality. |
| Quality Assurance Requirements | Month-to-month | Enhance the value of rigid, high-quality checks at every phase of manufacturing. |
| Sanitation Treatments | Every year | Keep high standards of health and sterility to guarantee product safety. |
| Security and Conformity | Every year | Make sure to adhere to worldwide security and compliance certifications. |
By purchasing the recurring advancement of the production team, derma roller dealers can cultivate a society of quality and duty. This not only boosts the general efficiency and integrity of the manufacturing procedure but additionally substantially decreases the risk of errors and non-compliance. Regular evaluations and comments systems must be applied to check the effectiveness of the training programs and make necessary changes.
Furthermore, partnerships with market specialists and participation in expert workshops and conferences can provide extra learning possibilities for staff. This outside exposure aids in comprehending international fads and ideal methods, which can be integrated into the business’s very own processes.
Inevitably, continuous training and education play a critical role in guaranteeing the top quality of derma rollers. By outfitting the production team with the necessary skills and knowledge, derma roller wholesalers can preserve high standards and meet the ever-evolving needs of the marketplace.
FAQs about Ensuring Quality in Derma Rollers
How do derma roller wholesalers ensure the quality of their products?
Derma roller wholesalers ensure quality by selecting high-quality raw materials, implementing rigorous manufacturing standards, conducting comprehensive quality control inspections, ensuring proper sterilization processes, adhering to international safety and compliance certifications, utilizing advanced packaging techniques, offering transparent supplier audits, and providing ongoing training and education for production staff.
What are the key components of ensuring quality in derma rollers?
The key components include selecting high-quality raw materials such as medical-grade stainless steel or titanium for needles, high-grade plastic or resin for the roller, and ergonomic, non-slip material for the handle. Implementing rigorous manufacturing standards, conducting quality control inspections, ensuring proper sterilization processes, adhering to safety certifications, utilizing advanced packaging techniques, and providing ongoing training for staff are also crucial components.
Why are supplier audits and documentation important for maintaining quality?
Supplier audits and documentation are important, as they help wholesalers verify the quality of raw materials, manufacturing processes, and compliance with standards. Detailed records of supplier audits ensure transparency and accountability, allowing for continuous improvement and risk mitigation.
How do wholesalers ensure the sterility of derma rollers?
Wholesalers ensure sterility by using appropriate sterilization methods such as gamma radiation, ethylene oxide gas, or autoclaving. They also implement advanced packaging techniques, including hermetic sealing, tamper-evident features, desiccants, and oxygen absorbers, to maintain sterility until the product reaches the end consumer.
What certifications should derma roller wholesalers adhere to?
Derma roller wholesalers should adhere to international safety and compliance certifications such as ISO 13485, CE Marking, and FDA clearance (510(k)). These certifications ensure that products meet quality, safety, and regulatory standards, allowing wholesalers to access global markets and build trust with consumers.
Why is ongoing training and education important for production staff?
Ongoing training and education are important for production staff to stay updated on industry practices, technological advancements, and regulatory requirements. This helps maintain high standards, reduce errors, and ensure compliance with quality control standards, leading to the production of high-quality derma rollers.